<!DOCTYPE HTML PUBLIC "-//IETF//DTD HTML//EN">
Polar 4.2 For Windows
Electrochemical
simulation and data analysis
DrHuang Pty Ltd
124 Eastern Avenue, Kingsford, Sydney, NSW 2032, Australia
Phone: (61 2) 9662 0516
mailto:info@electroanal.com
info@DrHuang.com
Copyright @ 1990-2000
October 14, 2000
Contents
Chapter
2 Polarography and Voltammetry
6.1.1
Effect of Electron Number
6.1.2
Effect of Electrode Geometry
6.4
Calculating Theoretical Limiting Current
6.5
Extracting Parameters by Curve Fitting.
6.5.1
Fitting to Simulation Curve
6.5.2
Fitting to Experimental Curve
Chapter
7 Frequently Asked Questions (FAQ)
<big>Chapter 1
Introduction</big>
Polar (an abbreviation for Polarography) is
a general electrochemical simulator. It analytically and digitally simulates
voltammograms (polarograms) on virtually any mechanism at 9 electrode
geometries (planar, spherical, semi-spherical, cylindrical, semi-cylindrical,
microdisc, thin film, and rotating electrodes) in over 5 techniques (linear
sweep and CV, DC, normal pulse, differential pulse, and square wave
voltammetries). It also simulates effects of charge current, resistance, noise,
electrolyte, stripping time, stripping potential, convection, etc. User can
type in his mechanism with any symbol.
It analyses any ASCII x-y data for detecting peak location, peak value,
semi-derivative, derivative, integral, semi-integral, curve fitting, and
separating overlapped peaks.
It shows tip when the user put mouse cursor over a label. The program can separate overlapped voltammograms into individuals, and extract real peak from voltammogram with noise and baseline. It outputs the theoretical peak values, the peak current and potential and current-potential data, which can be imported into other program (e.g. Lotus 123). User can copy-and-paste the voltammogram into his document.
It has been successfully applied to fit experimental polarograms (voltammograms) of In(III), Cd(II), Pb(II), Tl(I), Cr(III), Zn(II), and binuclear copper complex in aqueous and non-aqueous media at mercury, solid metal and non-metal electrodes (specifically the dropping mercury, hanging mercury drop, gold, platinum and glassy carbon electrodes) by various electrochemical techniques (differential pulse, square wave, and pseudo-derivative normal pulse polarographies) [1-5].
It is available from the author or my Web site. If you have any question, please read FAQ in its document. For tutorial, please read the course practices in the rmit.htm file.
It is assumed that you agree the Shareware license that you should register by US$20 with author in 20 days or you should delete it.
<big>Chapter 2
Polarography and
Voltammetry
</big>
2.1 Introduction
Modern
electrochemical methods offer the analytical chemist a wide variety of
techniques to solve analytical problems. Voltammetry is one such method, in
which the current is measured as a function of applied potential. Polarography
is another method, which differs from voltammetry in that it employs a dropping
mercury electrode (DME) to continuously renew the electrode surface.
In
this chapter, the fundamental principles of direct current polarography (DCP),
alternating current polarography (ACP), square wave polarography (SWP), normal
pulse polarography (NPP), differential pulse polarography (DPP), and
pseudo-derivative normal pulse polarography (PDNPP) are reviewed. Much of this
theory is also applicable to voltammetry. Linear sweep voltammetry (LSV) and
stripping voltammetry (SV) are also described. If you are familiar with
polarography and voltammetry, they can move directly to the next chapter.
Beside
techniques, theoretical equations also depend on mechanism, reversibility, and
electrode geometry. You can calculate the limiting current from the below
equations by clicking the Theoretical Peak submenu.
2.2 Direct
Current Polarography
Heyrovsky
invented the original polarographic method, conventional direct current
polarography (DCP), and Heyrovsky and Shikata constructed the first polarograph
in 1925 [6]. DCP involves the measurement of current flowing through the
dropping mercury electrode (DME) as a function of applied potential. Under the
influence of gravity, mercury drops grow from the end of a fine glass capillary
until they detach. Then the process is allowed to repeat itself. Drops may be
allowed to fall naturally or may be dislodged after a specified interval with
the aid of a mechanical device. A major advantage of the DME is that a
constantly renewed electrode surface is exposed to the test solution so that
problems of electrode blockage are avoided. Another advantage of the DME is
that it allows a number of electrode reduction processes to be monitored, which
would otherwise be inaccessible, because a wide negative potential region is
available on account of the high overpotential for water reduction.
If
an electroactive species is capable of undergoing a redox process at the DME,
then an S-shaped current-potential relation is usually observed. This is called
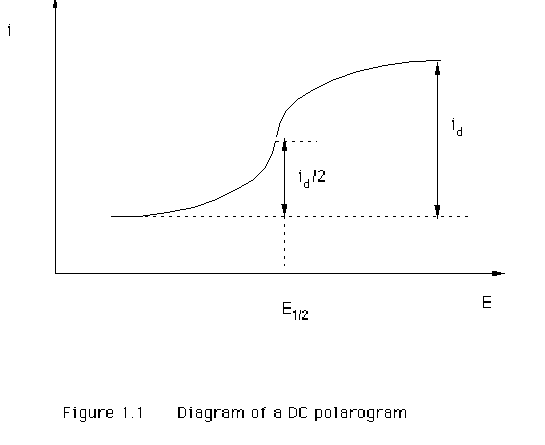
a polarographic wave. Figure 1.1 illustrates the response obtained from a
reduction reaction where the current (i) increases over a particular potential
(E) range until it reaches a limiting value. The limiting current is the
diffusion-controlled limiting current (id). This id is of interest in analytical measurements as it is
proportional to the concentration of reactant. Ilkovic [4] first put the
measurement of this current on a theoretical basis, and his equation is [4-6]
id = (7/3)1/2
(36 p)1/6 r2/3
nF D1/2 m2/3 td1/6 C (2.1)
where r
is the density of mercury, n is the number of electrons, F is Faraday's constant, D is the diffusion coefficient, m
is the flow rate of mercury, td is the drop time, and C is the concentration of the electroactive
species in the bulk solution.
For
a planar electrode,
id = nFAD1/2 C / (p td ) 1/2 (2.2)
For
a spherical electrode with radius r,
id = id(planar)+nFADC
/ r = nFAD1/2
C (1/ (p
td ) 1/2 + D1/2 / r ) (2.3)
For
a microelectrode, a steady-state current is
id = GnFA1/2 DC (2.4)
where G is an electrode geometry constant,
only depending on electrode geometry.
For
a microdisc electrode, G=4/(p) ˝
id =4/(p) ˝ nFA1/2 DC = 4nFDC r
For
a microsphere electrode, G=2p ˝
id = 2p ˝ nFA1/2 DC = 4pnFDC r
The
half-wave potential E1/2
is another important parameter of the DC polarogram. This is the potential at
which the current reaches half of its limiting value (Figure 1.1). The value of
half-wave potential is usually independent of concentration and is
characteristic of the electroactive species. Therefore it can be used for
qualitative characterization of the species, and is the foundation of
qualitative analysis.
The
shape of the DC polarogram is also very important to the overall characterization
of the electrode process. If the reduction reaction is reversible and
controlled by diffusion, the potential (E) is related to the concentrations of
reactant and product by the Nernst equation [7]:
E = E° + (RT/nF) ln( CO(0)/CR(0) ) (2.5)
where E° is the standard redox potential,
R is a gas constant, T is temperature, CO(0) and CR(0) are the surface concentrations of species Ox and
Red, respectively. The shape of the DC polarographic wave is then derived by
combining the Nernst and Ilkovic equations as follows [8, 9]
E = E1/2 + (RT/nF) ln( (id - i)/i )
or
i = id / [1 + exp( (nF/RT) (E - E1/2))] (2.6)
where
E1/2 = E° + (RT/2nF) ln( DR/DO ) (2.7)
Since
the diffusion coefficients of oxidized and reduced forms, DO and DR, are often almost equal, then E1/2 = E°. When i = id /2, then E = E1/2.
Equation
(2.6) is the Heyrovsky-Ilkovic equation, and is often used in investigations
into the nature of electrode processes. However, an experimental DC polarogram
also shows the oscillatory behavior of the current due to the growth and fall
of the mercury drop, and this is superimposed on the DC behaviour. This
invariably causes problems in the measurement of wave heights and/or half-wave
potentials, and of course has deleterious effects on measures of analytical
performance, especially sensitivity and resolution. Despite these problems, the
DME remains popular because of its constantly renewed surface.
2.3 Linear Sweep Voltammetry and Cyclic
Voltammetry
Linear
sweep voltammetry (LSV) is performed by applying a linear potential ramp in the
same manner as DCP. However, with LSV the potential scan rate is usually much
faster than with DCP. When the reduction potential of the analyte is
approached, the current begins to flow. The current increases in response to
the increasing potential. However, as the reduction proceeds, a diffusion layer
is formed and the rate of the electrode reduction becomes diffusion limited. At
this point the current slowly declines. The result is the asymmetric
peak-shaped I-E curve, as in Figure 1.3.
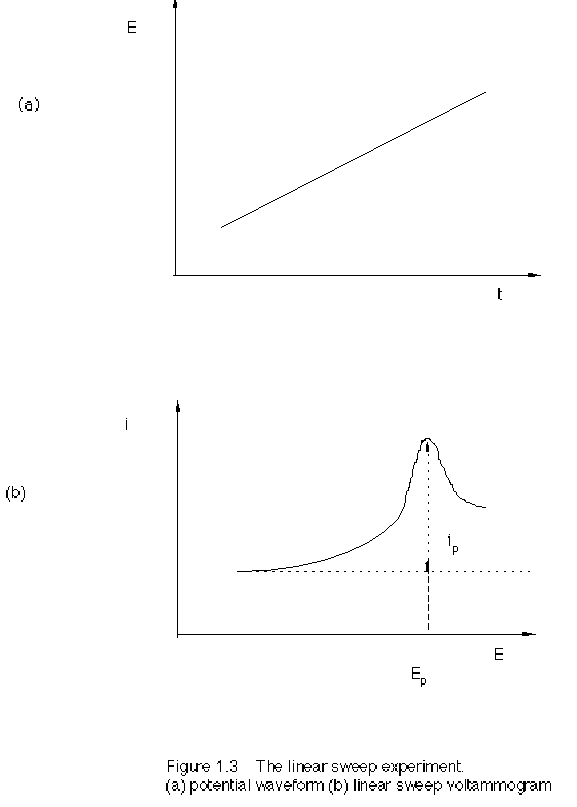
For a reversible reaction, the peak current is
Ip = 0.4463 nFAC (nFvD/(RT))1/2 (2.8)
The peak potential is
Ep = E1/2 – 1.109 RT/(nF) (2.9)
The half-peak potential is
Ep/2 = E1/2 + 1.09 RT/(nF) (2.10)
The difference between peak potential and half-peak potential, similar to the half-peak width, is
| Ep - Ep/2 | = 2.2 RT/(nF) = 56.5/n (mV) at 25 °C (2.11)
Cyclic voltammetry is similar to linear sweep voltammetry except for the potential scans from the starting potential to the end potential, then reverse from the end potential back to the starting potential. The difference between two peak potentials is
DEp =| Epa - Epc | = 2.3 RT/(nF) = 58/n (mV) at 25 °C (2.12)
For a non-reversible reaction, DEp becomes larger.
2.4 Alternating
Current Polarography
A
number of modifications to DCP have improved its analytical performance. One of
them is alternating current polarography (ACP). ACP is the result of
superimposing a small amplitude sinusoidal potential (DE) with a fixed frequency (w) on a slowly scanning DC ramp, as (c) in Figure 1.2.
The applied potential is then given by summing the AC and DC components.
Finally, the alternating current (AC) is measured as a function of DC
potential. In particular, the amplitude of the AC current vs. the DC potential
is plotted, as (g) in Figure 1.2. The current-potential (I-E) curve for a
reversible reaction follows the equation [6]
I = n2F2 AC
DE (wD)1/2 sech2
[(nF/2RT)(E - E1/2)] /(4RT) (2.13)
At a peak, sech()=1, then the above equation reduces
to
Ip = n2F2 AC DE (wD)1/2/(4RT) (2.14)
It
may be deduced from this equation that the amplitude of the AC component of the
Faradic current (I) is peak-shaped. Moreover, the peak current is a linear
function of concentration and therefore may be used in analytical applications.
Like the half-wave potential E1/2 in DCP, the peak potential Ep in ACP is characteristic of the electroactive
species. Also, the half-peak width (i.e. the width of the peak at half its
height, W1/2) is [6]
W1/2 = 3.52 RT/(nF) = 90/n mV at 25 °C. (2.15)
2.5 Square
Wave Polarography
Square
wave polarography (SWP) uses a small amplitude square wave voltage in place of
the sinusoidal one used in ACP. Its potential waveform is shown in (d) of
Figure 1.2. The current is sampled near the end of each square wave half cycle,
to minimize double-layer charging effects, and the I-E response is obtained by
plotting the differences in current between successive half cycles. For
reversible electrode processes, the I-E curve for SWP is similar to that in ACP
[6], so its properties, including the half-peak width W1/2 and resolution, are obviously akin to
ACP.
2.6 Normal
Pulse Polarography
The
pulse polarographies including normal pulse polarography (NPP) and differential
pulse polarography (DPP) stem from Barker's original work on square wave
polarography [6]. The increased sensitivity of these techniques over DCP arises
from their ability to discriminate against the charging current by measuring
the total current after the charging current has decayed to values
substantially less than the Faradic current.
The
potential-time waveform used in NPP is presented as (a) in Figure 1.2. At the
beginning of the potential sweep, the electrode is held at an initial potential
where no Faradic current flows.
Potential pulses of increasing amplitude are then applied to the
electrode at regular intervals. The potential pulses are about 50 ms in
duration and the current is measured at a time near the end of each pulse. A
potential pulse is ended by a return to the initial potential and the drop is
dislodged. The whole process is repeated except a few millivolts are added to
the potential pulse in next cycle. A normal pulse polarogram is shown as (e) of
Figure 1.2. The shape of the normal
pulse polarogram is sigmoidal, looking similar to the shape of a DC polarogram,
and indeed it can be described by a current-potential equation similar to that
in DCP [6].
For
a planar electrode,
id = nFAD1/2 C / (p tp ) 1/2 (2.16)
For
a spherical electrode with radius r,
id = id(planar)+nFADC
/ r = nFAD1/2
C (1/ (p
tp ) 1/2 + D1/2 /r ) (2.17)
2.7 Differential
Pulse Polarography
Normal
pulse polarography gives improved sensitivity by avoiding most of the charging
current by sampling the total current as late as possible after the application
of each potential pulse. However, there still is the charging current to some
extent. Another defect of NPP is poor resolution between neighbouring wave
because of drawn-out sigmoidal I-E response. Differential pulse polarography
(DPP) was designed to overcome these problems by arranging a charging current
of smaller magnitude, and by producing a peak-shaped I-E curve.
The
potential-time waveform used in DPP is shown as (b) of Figure 1.2. A voltage ramp
is applied to the electrode as in the DCP, and a small amplitude potential
pulse (DE) is added to the voltage towards the end of each drop's life. The
currents are measured before applying the pulse and at the end of the pulse.
When the difference between the two current samples is plotted as a function of
the applied ramp voltage, a peak-shaped current response is shown as (f) in
Figure 1.2.
The
peak-shaped I-E curve allows polarographic responses in close proximity to each
other to be more clearly resolved than in either DCP or NPP. The I-E curve for
all values of the pulse amplitude is described by [6]
I = nFAC (D/ p tp)1/2 P (s2-1)/[(s+P)(1+Ps)] (2.18)
where
s = exp(nFDE/(2RT)) (2.19)
P = exp[(nF/(RT))(E - E1/2 + DE/2)] (2.20)
At a peak, P=1, then the current equation reduces to
Ip = nFAC (D/ p tp)1/2 (s -1)/(1+s) (2.21)
Ep = E1/2 - DE/2 (2.22)
The half-peak width is a very important
parameter in resolution. The half-peak width W1/2 is a function of the pulse amplitude as follows [6]
W1/2 = 2RT/(nF) cosh-1[2 + cosh(nFDE/(2RT))] (2.23)
For large values of |DE| (say |DE| > 200/n mV), W1/2 approaches to |DE|, and for small values of |DE| (e.g. |DE| < 20/n mV), this
equation reduces to equation (2.15).
Unfortunately,
the above theoretical equations are derived by neglecting the DC effect in DPP,
and although this is not a problem when the ratio of the drop time to the pulse
time is larger than 50, the resulting distortion makes the theoretical
treatment complicated, especially for a non-reversible reaction.
2.8 Pseudo-Derivative
Normal Pulse Polarography
DPP
is a very sensitive electroanalytical technique due to the effective
discrimination against the charging current. However, DPP has two problems
associated with the slowly increasing DC ramp. As the DC ramp progresses,
filming may occur on the surface of the electrode if species form insoluble
mercury compounds [6]. Since the characteristics of the electrode are changed
by such a film, the current may not correspond to the simple theory. Another
problem is that the theory itself is complicated by the effect of the DC ramp.
NPP avoids these two problems. But the disadvantage of NPP is its poor
resolution because of the sigmoidal wave. To overcome this shortcoming, NPP
polarograms can be differentiated to produce peak-shaped responses, and thus
combine the best features of both DPP and NPP while avoiding some of their
limitations. This pseudo-derivative normal pulse polarography (PDNPP)
nevertheless is not sensitive as DPP.
The
potential-time waveform in PDNPP is as in NPP, but the current data of PDNPP
are displayed in a difference mode. The current is subtracted from those for
the following pulses, and the difference is plotted as a function of potential,
as in DPP.
The
theoretical treatment of PDNPP is simple and easy. The reversible
current-potential equation is similar to that of DPP except for the DC term
[6]. The half-peak width or resolution is akin to that of DPP.
2.9 Stripping
Voltammetry
Stripping
voltammetry involves three main steps: deposition (preconcentration),
equilibration, and stripping. The first step is to concentrate the analyte from
the dilute test solution into or onto the electrode at negative reduction (or
positive oxidation) potentials, usually accompanied by stirring. The second
step is to leave the solution to settle down. The third step is then to strip
the preconcentrated analyte from the electrode back into the solution by using
one of the polarographic techniques described above. A major advantage of this
method is its extremely sensitivity. This is because the concentration of the
analyte on the electrode is 100-1000 times greater than that in the starting
solution [6].
<big>Chapter 3
Features
This software analytically and digitally simulates voltammograms (polarograms) on virtually any mechanism at 9 electrode geometries in above techniques, calculates their theoretical peak current and potential, retrieve parameters by curve fitting, and separate overlapped peaks and baseline. </big>
·
Digital simulation
Flexible for any mechanism up to second-order chemical reaction. You can type
your mechanism and chemical symbols. An implicit finite difference algorithm.
·
Analytical simulation
No divergence problem in simulation. No overflow problem in simulation. Fast
simulation.
·
Over 5 techniques
Linear sweep, CV, DC, normal pulse, differential pulse, square wave
voltammetries. Multi-cyclic voltammetry, cyclic normal pulse, cyclic
differential pulse, cyclic square wave voltammetries.
·
Surface concentration
It shows what happen each species in the electrode surface.
·
Theoretical peak
You can compare your data with theoretical peak values to see if your
experimental conditions reach theoretical limit or not.
· Simulating effect of noise, charge current, resistance, electrolyte, stripping time, stripping potential, convection, etc.
·
Separating overlapped peaks
It manually and auto separates overlapped peaks into individuals, and extract
real peak from voltammogram with noise and baseline. So you can exactly
determine peaks.
·
Preconcentration
You can change preconcentration conditions for stripping voltammetry.
· Pre-equilibration
·
Curve fitting
It manually and auto fits the simulated voltammograms into experimental data,
and extracts kinetic parameters from experimental data.
·
Import and export data
You can export simulated data into your favor program (e.g. MS Excel). You can
copy-n-paste the voltammogram into your document.
·
Derivative, integral, semi-derivative, semi-integral
Semi-derivative is useful for CV. It can change a shape of reversible CV into
symmetric peak so easy to determine peak.
·
9 electrode geometries
planar, spherical, semi-spherical, cylindrical, semi-cylindrical, band,
microdisk, thin film, and rotating disk electrodes.
·
Tip
It shows tip for help when you put mouse cursor over a label.
Table 1 Feature
|
Version |
Shareware |
Student |
Standard |
Full |
Competitor |
|
digital simulation |
y |
y |
y |
y |
y |
|
analytical simulation |
y |
y |
y |
y |
n |
|
theoretical peak |
y |
y |
y |
y |
n |
|
multi-electron reaction |
y |
y |
y |
y |
n |
|
surface concentration |
y |
y |
y |
y |
n |
|
any species symbol |
y |
y |
y |
y |
n |
|
tip |
y |
y |
y |
y |
n |
|
Import and export data |
n |
y |
y |
y |
y |
|
Techniques: |
|
|
|
|
|
|
LSV and CV |
y |
y |
y |
y |
y |
|
DC |
y |
y |
y |
y |
n |
|
normal pulse |
y |
y |
y |
y |
n |
|
differential pulse |
n |
y |
y |
y |
n |
|
cyclic diff. pulse |
n |
y |
y |
y |
n |
|
square wave |
n |
y |
y |
y |
n |
|
Cyclic square wave |
n |
y |
y |
y |
n |
|
Effect: |
|
|
|
|
|
|
convection |
y |
y |
y |
y |
|
|
noise |
y |
y |
y |
y |
y |
|
charge current |
y |
y |
y |
y |
y |
|
resistance |
y |
y |
y |
y |
y |
|
electrolyte |
y |
y |
y |
y |
n |
|
preconcentration |
y |
y |
y |
y |
n |
|
pre-equilibration |
y |
y |
y |
y |
y |
|
Analysis: |
|
|
|
|
|
|
differentiate |
y |
y |
y |
y |
n |
|
integrate |
y |
y |
y |
y |
n |
|
Semi-differentiate |
y |
y |
y |
y |
n |
|
Semi-integrate |
y |
y |
y |
y |
n |
|
manual fit |
y |
y |
y |
y |
|
|
auto fit |
n |
n |
y |
y |
y |
|
manual separate |
y |
y |
y |
y |
n |
|
auto separate |
n |
n |
n |
y |
n |
|
Electrode: |
|
|
|
|
|
|
planar |
y |
y |
y |
y |
y |
|
(micro)spherical |
y |
y |
y |
y |
y |
|
(micro)hemispherical |
y |
y |
y |
y |
y |
|
(micro)cylindrical |
y |
y |
y |
y |
y |
|
(micro)hemicylindrical |
y |
y |
y |
y |
|
|
microdisc |
y |
y |
y |
y |
n |
|
band |
y |
y |
y |
y |
n |
|
thin film |
y |
y |
y |
y |
n |
|
rotating disc |
y |
y |
y |
y |
n |
Note: y = yes, n = no. Price may be changed.
<big>Chapter 4
Menu</big>
File menu
·
Save submenu
It saves experimental parameters.
·
Import Data submenu
It imports data from a file into Polar. The Plot window title will show the
file name of data.
·
Export Data submenu
It exports data to other program as data file. e.g. if you export data as
the .csv file, you open it into MS Excel by double-clicking it.
·
Copy To Clipboard
It copy graph into clipboard, so you can paste graph into your document.
·
Print
It prints graph.
· Exit
Input menu
· Technique submenu
Select one of 5 techniques. The default technique is LSV and CV.
· Mechanism submenu
Input your mechanism and species symbol on Digital simulation, or choose predefined mechanism on Analytical simulation. The default mechanism is Fe3+ + e = Fe2+.
· Instrument submenu
Change instrument parameters. You can use the default values without any change.
· Chemicals submenu
Input chemicals parameters. You can use the default values without any change.
Run menu
·
Simulate submenu
It runs simulation.
·
Manual Fit submenu
It fits simulated curve into experimental curve as you manually change
parameter values.
·
Auto Fit submenu
It auto fits simulated curve into experimental curve.
·
Manual Separate submenu
It separates overlapped peaks into individuals as you manually change parameter
values.
·
Auto Separate submenu
It auto separates overlapped peaks into individuals.
Display menu
·
Option submenu
It is to change plot options.
·
Plot submenu
It plots curve without run simulation.
·
Next submenu
It plots next curve.
· Time
Display the simulation time and curve-fitting time.
· Clear submenu
Clear the Result window.
Analyze menu
· Find Peak submenu
Find the peak current and potential of curves of the peak shape.
· Find halfwave E submenu
Find the halfwave potential and limiting current of curves of the S shape.
· Theoretical Peak submenu
Calculate the theoretical limiting current, peak current and potential from theoretical equations. You select a mechanism from Analytical Simulation in the Mechanism window.
· Semi-differentiate submenu
Deconvolution.
· Semi-integrate submenu
Convolution.
· Differentiate submenu
· Integrate submenu
· X Data submenu
Multiply 10, 1000, 0.1, or 0.001 on X data. If your experimental potential data is not in Volt unit, you should convert to Volt unit by this submenu.
X data reverse submenu
Reverse the order of data.
· Y Data submenu
Multiply 10, 1000, 0.1, or 0.001 on Y data. . If your experimental current data is not in Amp unit, you should convert to Amp unit by this submenu.
Help menu
·
Logon submenu
You logon to activate menus by input of password.
·
Manual submenu
It displays this manual.
Some menus will be activated only after you click the Simulate submenu or load data because they require data.
<big>Chapter 5
Input</big>
5.1 Techniques window
1) Linear sweep and cyclic voltammetry
2) DC voltammetry
3) Normal pulse voltammetry and cyclic normal pulse voltammetry
4) Differential pulse voltammetry and cyclic differential pulse voltammetry
5) Square wave voltammetry and cyclic square wave voltammetry
The shapes of DC and normal pulse polarogram are S-shape. The shapes of differential pulse and square wave voltammograms usually are peak-shape. But there is effect of the DC term on differential pulse voltammogram.
5.2 Mechanism window
You can type in your mechanism in Digital Simulation section by any symbol. In order to faster computation, you should type in reactants only without products if chemical reaction is irreversible. In Analytical Simulation, you choose a predefined mechanism.
5.3 Instrument Window
Instrumental Parameters Section:
E start: starting potential (V).
E end: ending potential (V).
E step: step potential (V).
v: scan rate (V/s). For square wave voltammetry, v=E step/t pulse.
E pulse: pulse potential (V).
T: temperature (°C).
t pulse: pulse time or pulse width for pulse voltammetry (s).
t drop: mercury dropping time or pulse length in pulse voltammetry (s).
Noise: ratio of noise to maximum signal (%).
Scan:
Single: single scan.
Cycles: cyclic scan, e.g. cyclic
voltammetry (CV).
2 Cycles: 2-cycle scan.
Electrode Section:
Planar: planar electrode.
(Micro)Spherical: spherical
electrode or micro spherical electrode.
(Micro)Hemispherical: hemispherical
electrode or micro hemispherical electrode.
(Micro)Cylindric: cylindrical
electrode or micro cylindrical electrode.
Microdisc: microdisc electrode,
radius <1e-4 cm.
Thin film: thin film electrode.
Rotating disc: rotating disc
electrode with convection.
Area: electrode area (cm2).
When you change the value of area, the value of radius is changed
automatically.
Radius: electrode radius (cm). When you change the value of radius, the
value of area is changed automatically.
Length: electrode length for cylindrical electrode or micro cylindrical
electrode, or mercury film thickness for stripping voltammetry (cm).
Rotation: electrode rotation rate (rpm).
Preconcentration Section:
E pre: preconcentration potential (V).
R stir: stirring rate (rpm).
Stirring solution.
t pre: preconcentration time (s).
t pre const: preconcentration time
constant (/s).
P const: electrode constant. It only
related to electrode.
Baseline section:
C dl: double layer capacitor for charge current (F).
R: resistance (Ohm).
I start: a starting current (A).
I end: an ending current (A).
5.4 Chemicals Window
Pre-equilibration option:
When this option is enabled, it automatically assumes that all the chemical and electrochemical reactions in the vicinity of the electrode surface are in equilibrium as determined by the thermodynamic parameters: chemical equilibrium constant Keq, the standard potential E°, and by the starting electrode potential Estart. Then, the entered values of analytical concentrations are not identical to the corresponding initial concentrations.
It is a good idea to keep the pre-equilibration option enabled. When the pre-equilibrated and analytical concentrations are different significantly, the initial condition for the experiment and the simulation may not be what was expected. The degree, to which the pre-equilibrated concentrations may be considered to be the bulk concentrations, will depend upon time of pre-equilibration (i.e., the time between setting the starting potential and initiating the potential scan), the operative kinetics, and the geometry. The value of the initial concentrations will act as if they are the bulk concentrations. A reasonable assumption only if the electrode geometry does not produce steady-state diffusion and if the pre-equilibration time is much longer than the duration of experiment.
When the pre-equilibration is not selected, the pre-equilibrated and analytical concentrations are the same.
Species Section:
D:
diffusion coefficient (cm2/s).
C anal: analytical concentration
(M).
C init: initial concentration for
simulation (M).
C fitted: fitted value of concentration
(M).
C min: minimum concentration for
fitting (M).
C max: maximum concentration for
fitting (M).
Heterogeneous Reaction Section:
ks:
heterogeneous standard rate constant (cm/s).
a:
electron transfer coefficient.
n: electron number.
E°: standard electrode potential (V).
Homogeneous Reaction Section:
kf:
forward chemical reaction rate constant.
kb: backward chemical reaction rate
constant.
Keq: chemical equilibrium constant,
Keq = kf / kb.
<big>Chapter 6
Playing Around</big>
6.1 Running Simulation
A simplest way to run simulation is just to click the Run menu and then the Simulate submenu. It uses the default values to simulate a linear sweep voltammogram. You can change chemical parameters in the Chemical window under the Chemical menu, or change instrumental parameters in the Instrument windows under the Instrument menu, or change mechanism in the Mechanism window under the Mechanism menu.
Notice that some menu (e.g. the Display menu and the Analysis menu) will be activated only after run simulation or load data because they require data.
6.1.1 Effect of Electron Number
Change the electron number of charge reactions in Digital Simulation section for both Digital and Analytical Simulation. Peak height increases and peak width decreases as the electron number increases.
6.1.2 Effect of Electrode Geometry
Simulation technology for microelectrode is the same as for macro electrode, but the electrode size is very small, e.g. electrode radius is 1e-4 cm. A shape of linear scan voltammogram at spherical electrode is changed from peak shape to S-shape. When the products of scan rate and radius, vr > 10-5, the shape is peak. When vr < 10-7, the shape is wave. Note that the planar electrode geometry is not available for microelectrode.
The rotating disc electrode is similar to spherical electrode. When the ratio of rotating speed to scan rate, w/v < 1, the shape is peak. When w/v > 103, the shape is wave.
6.2 Comparing Curves
After run first simulation, click the Display menu, and then the Option submenu. Select the Overlap choice, and then run second simulation.
6.3 Analyzing Data
Semi-derivative is useful for CV. It can change a shape of CV into symmetric peak for easy measurement.
6.4 Calculating Theoretical Limiting Current
Click the Analyze menu and then the Theoretical Peak submenu to calculate the values of theoretical limiting current, peak current and potential. Change a mechanism for the theoretical peak from the Analytical Simulation section in the Mechanism window.
6.5 Extracting Parameters by Curve Fitting
6.5.1 Fitting to Simulation Curve
In order to extract kinetic parameters, you can fit a simulation curve to another simulated or experimental curve. It can retrieve any of 30 parameters (eg. concentration C, standard electrode potential E°, and the heterogeneous standard rate constant ks) from voltammogram by curve fitting. You select a parameter that you want to fit, input the minimum and maximum values of the parameter. e.g. after run simulation with all default values, select a concentration, then change the C value from 1e-3 to 2e-3 in the Species section, click the Auto Fit menu. You will see the fitted value of 0.001 in the C fitted field next to the C text field.
You should manual fit before auto fit. The manual fit shows how well your initial guesses values work. . If it diverged, you should change their initial values, then try again.
6.5.2 Fitting to Experimental Curve
It is similar to fit simulated curve. But you should input your experimental values of Estart, Eend, Estep, etc. into the Experimental section. Polar requires that data are in SI unit and first peak is positive value. If your experimental data are not, please convert your experimental data. E.g. click the Analyze menu, and then the 0.001Y submenu to convert current from mA to A.
6.6 Stripping
Voltammetry
Select the
Preconcentration in the Experiential Parameters window. Change the
preconcentration potential value in the E pre text field, and
preconcentration time in the t pre text field. The preconcentration
potential value usually is -0.2/n V to specie’s standard electrode potential.
The preconcentration time usually is a number of minutes. You should enter your
electrode constant into the P cont text field, and your mercury film
thickness into the Length field in the Electrode section of the
Experimental window if you use a planar mercury film electrode.
<big></big>Chapter 7
Frequently Asked Questions (FAQ)
Q: Which
platforms can Polar run on?
A: Its 32-bit version Polar runs on IBM PC under
Windows 95/98/NT while its 16-bit version Polar runs under Windows
3/3.1/3.11/95/98/NT.
The 32-bit version needs Microsoft Visual Basic 6 runtime DLL files (e.g. msvbvm60.dll, comdlg32.ocx) in the same directory as Polar or in the directory \windows\system for Windows 3.11 or 95, or in the directory \winnt\system32 for Windows NT.
The 16-bit version needs Microsoft Visual Basic 4 runtime DLL files (e.g. vb40016.dll and oc25.dll) in the same directory as Polar or in the directory \windows\system for Windows 3.1, or in the directory \winnt\system for Windows NT.
Q: I cannot save a file.
A: You miss the Microsoft Visual Basic 6 runtime DLL file comdlg32.ocx.
Q: Where can I download these dll?
A: Microsoft Visual Basic 6 runtime DLL files are from http://www.simtel.net/simtel.net/win95/dll.html, where msvbvm60.dll is inside simvb6-5.zip. Microsoft Visual Basic 4 16-bit runtime DLL files are from http://www.simtel.net/simtel.net/win3/dll.html.
Q: When I click
the Simulate menu, I got error: “No data”, or "Run-time error 13”,
with the message: "Type mismatch".
A: I guess you are running it under non-English version of Windows. Please change language setting to English in the Regional Setting of the Control Panel, and restart Polar. Or try it under English version of Windows. Some non-English versions of Windows have problem to run English version program.
Q: When I
installed to run setup.exe, an error occurred:
while registering the file
>c:\windows\system\MSRD2x35.dll
Shall I (Abort, Retry, Ignore)?
A: Ignore. Do not worry about MSRD2x35.dll. Running Polar did not use it,
setup.exe check it only.
Q: Still have
install problem?
A: You should close all programs (include Office, Mail)
before install Polar. If you still have problem, try to register file
msvbvm60.dll by double click or type following command in DOS:
Cd \windows\system
Regsvr32 msvbvm60.dll
then start Polar.
Q: Why are some
menus inactive?
A: Some menus will be activated only after you click
the Simulate menu or load data because they need data.
Q: I cannot see
any chemical reaction in Public version. Is this part of the program not
finished yet or is it only available in the registered version?
A: There is only one chemical reaction available in the
Public version. You can change chemical reaction rate kf, e.g. 1010.
The registered versions simulate virtual any mechanisms.
Q: Does it
include my mechanism?
A: If your mechanism is missing, please send your
requirement into author. Author may add your mechanism into new version special
for you.
Q: Can it fit
data by curve fitting?
A: Yes. Click to select a parameter that you want to
fit, and then click the Auto Fit menu.
Q: Can I change
graph into other program Lotus 123 or Excel?
A: Yes. You export data in text file, and then read
data into Lotus 123 or Excel.
Q: Some
submenus semi-derivative, semi-integral, derivative, and integral, seem to not
work sometime. How can I do?
A: You should first click the Next submenu under the
Plot menu, then try semi-derivative submenu.
Q: How much
does registration cost?
A: About $100.
Q: How can I
get registered version?
A: You will receive it if you send author register fee
by check or money order.
Q: What are
differences among Shareware, Standard and Professional, and Full versions?
A: The Shareware version is for teaching, the Standard
version is for average users, the Professional version is for professionals,
and the Full version is for special users.
Q: When I run
the SWV with default conditions as a digital simulation, it does not appear to
give the correct curve. Why?
Because default conditions are for linear sweep and CV
only. For SWV, DC, NPV and DPV, you should change scan rate v to 0.01. For SWV
you should calculate correct scan rate by v=E step/t pulse before run digital
simulation.
Q: Is it possible to click on a point and then have
displayed both the current and potential for the point? Also, does it have any
zoom features?
A: You can import any ASCII x-y data from Polar to
my software VisualMath For Java. In VisualMath For Java, you click on a point
and then have displayed both the current and potential for the point. Also, it
has zoom features.
Q: How to
simulate oxidation reaction?
A: change the Estart < the Eend in the Instrument window.
Q: How does it
compare to competitors?
A: Polar has advantages over competitors:
1. Competitor simulates a single technique CV only; while Polar has more features, e.g. Polar simulates over 5 techniques (see details on the table in Chapter 2 Features).
2. Competitor cannot separate overlapped peaks, while Polar does.
3. Competitor does not support Windows 95 features, e.g. long filename, while Polar does.
4. Competitor cannot simulate multi-electron reaction in one step, while Polar does.
5. You download and try Polar free.
6. Polar is much cheaper.
7. You do not worry about if you lose the Dongle. Competitor is copy-protected by the Dongle, but Polar is not.
<big>Chapter 8
References</big>
[1] W. Huang, T. Henderson, A.M. Bond and K.B. Oldham, Curve fitting to resolve overlapping voltammetric peaks: model and examples, Anal. Chim. Acta, 1995, 304, 1-15.
[2] W. Huang and B. Hibbert, Computers & Chem., 1995, 19(4), 433.
[3] W. Huang and B. Hibbert, Computers & Chem., 1995, 19(4), 435.
[4] W. Huang and B. Hibbert, Polar 2.0 for Windows: simulator of voltammogram, Chem. in Aus., 1996, 131.
[5] J. Mo, P. Cai, W. Huang and F. Yun, Theory and application on multiple semidifferential electrochemical stripping analysis with thin mercury film formed in situ, Acta Chimica Sinica, 1984, 42(6), 556-561, [CA 101: 162712].
[6] A. J. Bard and L. R. Faulkner, Electrochemical Methods, John Wiley & Sons, New York, 1980.