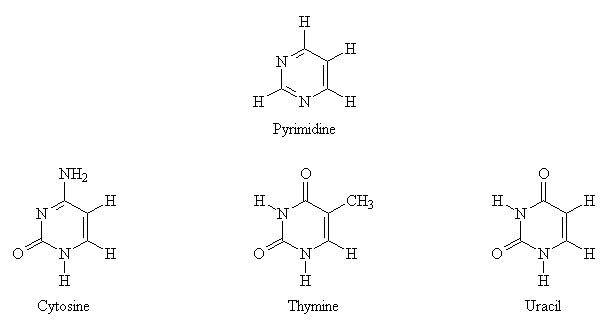
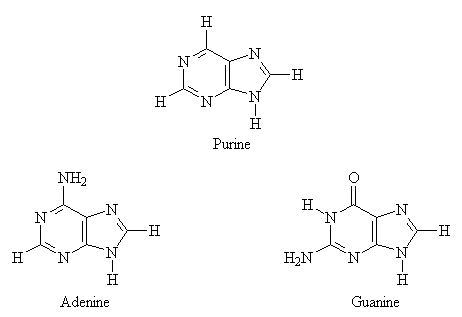
DNA and RNA contain bases derived from pyrimidine (a nitrogen-base containing a single aromatic ring) and purine (a nitrogen-base containing two fused aromatic rings). The structures of both the parent bases and the derivatives commonly found in nucleic acids are shown below.


One of the most important structural features of polynucleotides is the fact that they are able to participate in H-bonding. The diagram below indicates the H-bond donor and H-bond acceptor sites for the common nucleic acid bases.
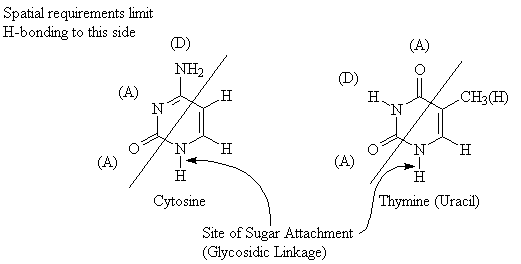
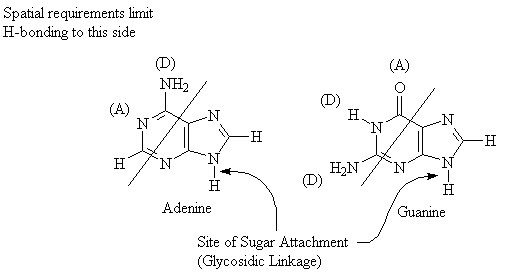
Steric/spatial requirements in DNA require that a single pyrimidine interacts with a single purine base. Two pyrimidine bases are too small, and cannot get close enough together to form H-bonds. Conversely, two purine bases are too large to fit into the gap separating the two strands of DNA.
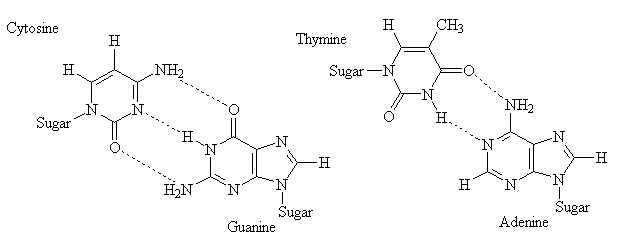
The above diagram illustrates the Hydrogen-bonding pairs found in DNA. Note that the A-T pair has only two H-bonds because of the adenine molecule. (The lone pair of electrons on the oxygen atom in thymine molecule could act to form a third H-bond, but adenine does not have the required donor). The arrangement of donors and acceptors in thymine and guanine precludes formation of H-bonding pairs between these two compounds.
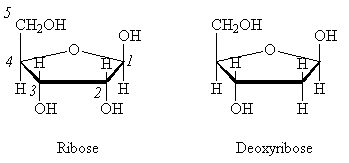
Ribose and deoxyribose are the sugars found in RNA and DNA, respectively. The only difference in structure between these two molecules occurs at C2.
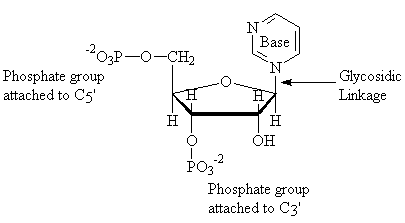
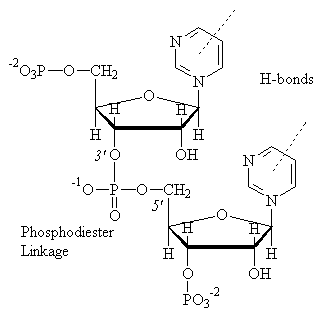
Shown above is a nucleotide, which consists of a base, a sugar, and a phosphate group, with a second phosphate group attached to C3. Shown below is a poly-nucleotide, linked by a phosphodiester bond.
Last modified February 25, 1997
Kent State University - Stark Campus
Department of Chemistry
This Page Written and Maintained by Dr. Clarke Earley