
Introduction to Molecular Approaches of Density Functional Theory
Jan K. Labanowski (jkl@ccl.net)
Ohio Supercomputer Center, 1224 Kinnear Rd, Columbus, OH 43221-1153
Performance of DFT
This will be a very short list of DFT applications, as there are many excellent reviews on this topic
(see e.g.: Labanowski & Andzelm, 1991; Parr & Yang, 1989; Seminario & Politzer 1995; Ziegler, 1991; Bartolotti and Flurchick, 1996; St-Amant. 1996).
The G1 database of Pople and coworkers is a benchmark for accuracy of the traditional ab initio methods.
The database containes 55 molecules for which experimental values of atomization energies are known within
1 kcal/mol. Curtiss et al (1991) achieved the 1.2 kcal/mol mean absolute error for these 55 atomization
energies using the G2 procedure, which is a quite involved prescription incorporating higher order correlated methods.
Becke (1992) was able to reproduce values in this database with a mean absolute error of 3.7 kcal/mol using his NUMOL
program with
gradient corrected functionals. This result was additionally improved by Becke (1993) to 2.4 kcal/mol when portion of
the electron exchange entering the echange-correlation energy, 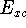 was calculated exactly form Kohn-Sham orbitals
While the error in DFT is considered still too big, these results were obtained with a method
which is substantially less computationally demanding than
original correlated ab initio procedures used by Pople and coworkers. Obviusly, the errors refer to absolute
atomization energies, which in general are very difficult to calculate with good occuracy
(for review see, e.g., Hehre et al, 1986). The relative differences are usually reproduced much better
with DFT methods.
was calculated exactly form Kohn-Sham orbitals
While the error in DFT is considered still too big, these results were obtained with a method
which is substantially less computationally demanding than
original correlated ab initio procedures used by Pople and coworkers. Obviusly, the errors refer to absolute
atomization energies, which in general are very difficult to calculate with good occuracy
(for review see, e.g., Hehre et al, 1986). The relative differences are usually reproduced much better
with DFT methods.
Even without gradient corrections DFT results for bond dissociation energies are usually much better then the Hartree-Fock (which routinely underbinds) results, though they have an overbinding tendency. The LDA results are approximately of MP2 quality. The inclusion of gradient corrections to DFT provides bond dissociaction energies which pair in occuracy with MP4 and CC results.
Molecular geometries even with LSD are much better than corresponding HF results and are of the MP2 quality. However, LSD fails to correctly treat hydrogen bonding. This deficiency is removed when one uses gradient corrected DFT approaches. Quite estonishingly, DFT provides excellent results for molecules which are notoriously difficult for traditional ab initio approaches like FOOF, FON, and metalorganic or inorganic moities. There seems to be a funny regularity: "If something does not work with ab initio, try it with DFT, and vice versa".
Transition states of organic molecules are frequently not reproduced well with "pure" DFT. However, it seems that admixture of exact exchange (see Becke, 1993) via ACM dramatically improves the problem cases. DFT is however a metod of choice for transition states in metalorganic reactions. These systems are notoriously difficult to treat with even high quality ab initio and have problems with convergence.
Vibrational frequencies are well reproduced even by LSD, though gradient corrections improve agreement with experiment even further.
Ionization potentials, electron affinities, and proton affinities are reproduced fairly well within gradient corrected DFT.
Recently, there is much interest in using DFT for high spin species, since preliminary results are very promissing. On the other hand good performance of DFT in this field comes as a surprise, since high multiplets are poorly described by a single determinant wave function. For all the wrong reasons, with KS wave function of broken symmetry, the multiplets splitting are quite well reproduced by DFT.
The scope of applcations for DFT grows rapidly with calculations of new molecular properties being added to actively developed software. Recent extensions include parameters for NMR and ESR spectroscopy, diamagnetic properties, polarizabilities, relativistic calculations, and others.
The DFT methodology is still far from mature. It is encouraging that several groups which were primarily focused on the development of traditional ab initio approaches are now actively working in the DFT area. It will bring new methodological developments, as well as, more carefull assesment of applicability. With all the hopes which DFT brings, one has to keep in mind, that it is primarily a ground state oriented method, and cannot compete with semiempirical and correlated ab initio methods in calculations concerning excited states.
