
Next: About this document Up: No Title Previous: Concluding Remarks
References
- (1)
-
Dixon D.A.; Lias, S.G. In Molecular Structure and Energetics, Vol. 2,
Physical Measurements; Liebman, J. F.; Greenberg, A., Eds.,
VCH, Deerfield Beach, FL, 1987.
- (2)
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; Pople, J.A.
Ab Initio
Molecular Orbital Theory; John Willey and Sons, New York, 1986.
- (3)
- Ozment, J. L.; Schmiedekamp, A. M. Int. J. Quantum Chem.
1992, 43 783.
- (4)
-
Parr, R.G.; Yang W. Density Functional Theory of Atoms and Molecules;
Oxford University, New York, 1989.
- (5)
-
Density Functional Methods in Chemistry; Labanowski, J. K.;
Andzelm, J.W., Eds., Springer Verlag, New York, 1991.
- (6)
- Ziegler, T. Chem. Rev. 1991, 91, 651.
- (7)
- we used the following constants:
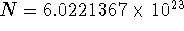 molecules/mol,
1 J
molecules/mol,
1 J  4.184 cal; R = 1.9872156 cal mol
4.184 cal; R = 1.9872156 cal mol  K
K  ;
h = 6.6260755
;
h = 6.6260755  J s;
1 cm
J s;
1 cm 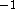 = 0.0028591439 kcal mol
= 0.0028591439 kcal mol 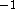 ;
1 hartree = 627.50955 kcal mol
;
1 hartree = 627.50955 kcal mol 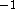 ;
1 bohr = 0.529177249 Å.
;
1 bohr = 0.529177249 Å.
- (8)
- Gaussian90, Revision H; Frisch, M. J.; Head-Gordon, M.;
Trucks, G. W.; Foresman, J. B.; Schlegel, H. B.;
Raghavachari, K.; Robb, M.; Binkley, J. S.; Gonzalez, C.;
Defrees, D. J.; Fox, D. J.; Whiteside, R. A.; Seeger, R.;
Melius, C. F.; Baker, J.; Martin, R. L.; Kahn, L. R.;
Stewart, J. J. P.; Topiol, S.; Pople, J. A.
Gaussian Inc., Pittsburgh PA, 1990.
- (9)
- Stanton, J. F.; Gauss, J.; Watts, J. D.; Lauderdale, W. J.;
Bartlett, R. J. ACES2 quantum package, Quantum Theory Project of the
University of Florida at Gainesville.
- (10)
- Dunning, T.H.; Hay, P.J. In Modern
Theoretical Chemistry vol. 3; Schaefer III, H.F., Ed.,
Plenum Press, New York, 1977; pp 1-28.
- (11)
- These basis sets differ
from the D95H** set supplied with Gaussian90 in the values of exponents at
polarization functions.
- (12)
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.,
Schleyer P.v.R. J. Comp. Chem. 1983, 4, 294.
- (13)
- Del Bene, J. E. J. Phys. Chem. 1993, 97, 107.
- (14)
- Andzelm, J. In Density Functional Methods
in Chemistry; Labanowski, J.K.; Andzelm, J.W., Eds.,
Springer-Verlag, New York, 1991; pp 155-174.
- (15)
- DMol: Local Density Functional Program, Ver. 2.0; Biosym
Technologies, San Diego, CA.
- (16)
- St-Amant, A., Ph.D. thesis, Université de Montrèal, 1992;
St-Amant, A.; Salahub, D. R. Chem. Phys. Lett.
1990, 169, 387.
- (17)
- Vosco, S.H.; Wilk, L., Nuisar, M. Can. J. Phys. 1980,
58, 1200.
- (18)
- von Barth, U.; Hedin, L. J. Phys. C 1972,
5, 1629.
- (19)
- Huzinaga, S.; Andzelm, J.; Klobukowski, M.;
Radzio, E.; Sakai, Y.; Tatewaki, H. Gaussian Basis Sets for Molecular
Calculations; Elsevier, Amsterdam, 1984.
- (20)
- Godbout, N.; Salahub, D. R.; Andzelm, J.; Wimmer, E.;
Can. J. Chem. 1992, 70, 560.
- (21)
- Sybyl Molecular Modeling Software, Ver. 5.3, Tripos Associates,
St. Louis, MO.
- (22)
- von Nagy, E.I.; Kimura, K.
J. Phys. Chem. 1990, 94, 8041.
- (23)
- Marcoccia, J.F.; Csizmadia, I.G.; Peterson, M.R.; Poirier,
R.A. Gazz. Chim. Ital. 1990, 120, 77.
- (24)
- Harmony, M. D.; Laurie, V. W.; Kuczkowski, R. L.;
Schwendeman, R. H.; Ramsay, D. A.; Lovas, F. J.; Lafferty, W. J.;
Maki, A. G.; J. Phys. Chem. Ref. Data 1979, 8, 619.
- (25)
- Callomon, J. H.; Hirota, E.; Kuchitsu, K.;
Lafferty, W. J.; Maki, A. G.; Pote, C. S. Structure Data of Free
Polyatomic Molecules; Hellwege, K.-H.; Hellwege, A. M., Eds.;
Landolt-Bornstein, Group II, New Series, Vol. 7, 1976.
- (26)
- Sasada, Y.; Takano, M.; Satoh, T.
J. Molec. Spectr. 1971, 38, 33;
Sasada, Y. J. Mol. Spectr. 1988, 190, 93.
- (27)
- Blake, J.; Tirado-Rives, J.; MindTool for SunOS, ver.
March 92. Available from anonymous ftp on rani.chem.yale.edu and
www.ccl.net.
- (28)
- Hill, R.A.; Labanowski, J.K.; Heisterberg, D.J.;
Miller, D.D. In Density Functional Methods
in Chemistry; Labanowski, J.K.; Andzelm, J.W., Eds.,
Springer-Verlag, New York, 1991; pp 357-372.
- (29)
- Becke, A.D. Phys. Rev. A 1988, 38, 3098.
- (30)
- Perdew, J.P. Phys. Rev. B 1986, 33, 8822;
erratum ibid. 1986, 38, 7406.
- (31)
- Sim, F.; St-Amant, A.; Papai, I.; Salahub, D.R.
J. Am. Chem. Soc. 1992, 114, 4391.
- (32)
- Masamura, M. Theor. Chim. Acta
1989, 75, 433.
- (33)
- McQuarrie, D. A.; Statistical Mechanics
Harper & Row, New York, 1976.
- (34)
- Lias, S.G.; Liebman, J.F.; Levin, R.D. J. Chem. Phys.
Ref. Data 1984, 13, 695; and updates available from the
NIST Web site: http://webbook.nist.gov/.
- (35)
- Del Bene, J.E.; Frish, M.J.; Raghavachari, K.; Pople, J.A.
J. Phys. Chem. 1982, 86, 1529.
- (36)
- Siggel, M.R.F.; Thomas, T.D.; Saethre, L.J.
J. Am. Chem. Soc. 1988, 110, 91.
- (37)
- Callomon, J. H.; Hirota, E.; Iijima, T.; Kuchitsu, K.;
Lafferty, C. S.; Pote, C. S. Structure Data of Free
Polyatomic Molecules; Hellwege, K.-H.; Hellwege, A. M., Eds.;
Landolt-Bornstein, Group II, New Series, Vol. 15, 1986.
- (38)
- Benston O.J., Ewbank J.D., Paul D.W., Klimkowskim V.J., Schaefer L.
Appl. Spectr. 1984, 38, 204.
- (39)
- D. Davies R. W., Robiette A.G., Gerry M.C.L., Bjarnov E., Winnewisser G.,
J. Mol. Spectr. 1980, 81, 93.
- (40)
- Salahub, D.R.; Fournier, R.; M
 ynarski, P,;
Papai, I.; St-Amant, A.; Ushio, J. In Density Functional Methods
in Chemistry; Labanowski, J.K.; Andzelm, J.W., Eds.,
Springer-Verlag, New York, 1991; pp 77-100.
ynarski, P,;
Papai, I.; St-Amant, A.; Ushio, J. In Density Functional Methods
in Chemistry; Labanowski, J.K.; Andzelm, J.W., Eds.,
Springer-Verlag, New York, 1991; pp 77-100.
- (41)
- Bartmes, J.E.; McIver Jr., R.T. In Gas Phase Ion Chemistry,
vol 2; Bowers, M.T., Ed., Academic Press, New York, 1979; pp 87-121.
- (42)
- Oie, T,; Topol, I.A.; Burt, S.K.; J. Phys. Chem.
1994, 98, 1121.
- (43)
- Chen, H.; Krasowski, M.; Fitzgerald G.;
J. Chem. Phys. 1993, 98, 8710.
Next: About this document Up: No Title Previous: Concluding Remarks Computational Chemistry
Thu Oct 30 00:15:06 EST 1997
