Chemical Structures of Biomolecules Chemical Structure of
Biologically Important Compounds
This page gives the chemical structures of several molecules commonly encountered in biological processes (particularly the Kreb's cycle). The structures of all of these molecules contain the same adenosine nucleotide core:
- Base: Adenine
- Sugar: Ribose
- Phosphate: PO4 group on Carbon-5 of ribose.
The molecules included on this page are:
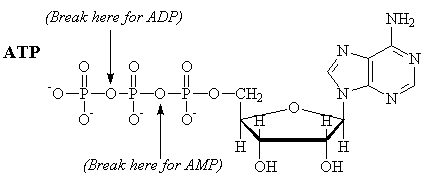
ATP
Adenosine triphosphate is a molecule commonly used by the body to provide energy. Formally, the energy comes from the following hydrolysis reaction, which produces the diphosphate (ADP). Note that "free" phosphate is also produced. Typically, the actual reactions used in biological processes are phosphorylation reactions, where a phosphate group is transfered from ATP to another compound.
ATP-4 + H2O ¾®
ADP-3 + HPO4-2 + H+
ATP + H2O ¾® ADP +
Pi + H+
DG = -7.3 kcal/mole
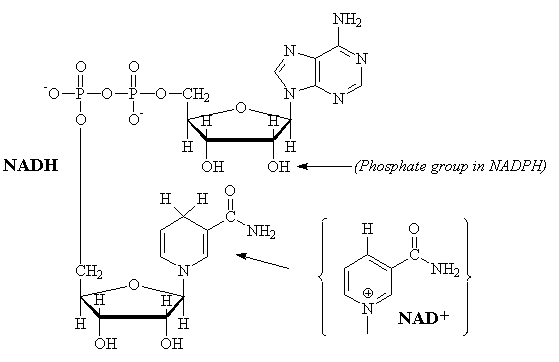
NAD
The interconversion of nicotinamide adenine dinucleotide between the reduced (NADH) and oxidized (NAD+) forms is a common reaction in biological redox (oxidation-reduction) reactions. Two electrons are transferred in the reaction. The NADH produced by the Kreb's cycle is oxidized by the respiratory cycle to produce 3 equivalents of ATP. In many anabolism reactions, the structurally similar NADPH/NADP+ redox couple is used.
NADH-2 ¾® NAD-1
+ H+ + 2e-
NADH ¾® NAD+ + H+
+ 2e-
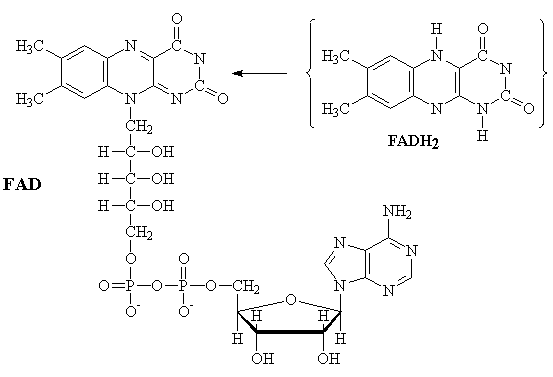
FAD
The interconversion of Flavin adenine dinucleotide between the reduced (FADH2) and oxidized (FAD) forms is an alternative to the NADH/NAD+ couple in biological redox (oxidation-reduction) reactions. Two electrons are transferred in the reaction. One important difference between these two couples is that the FADH2 produced by the Kreb's cycle is oxidized by the respiratory cycle to produce only 2 equivalents of ATP.
FADH2-2 ¾®
FAD-2 + 2H+ + 2e-
FADH2 ¾® FAD + 2H+
+ 2e-
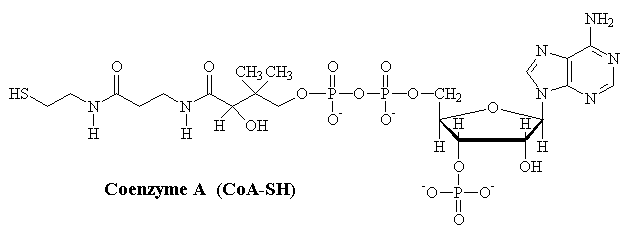
Coenzyme A
Coenzyme-A (CoA) is an important catalyst for the activation of organic molecules. Perhaps the most commonly encountered example of this reactivity is in the activation of pyruvate to acetyl-CoA, used in preparation for the Kreb's cycle. The key feature of this reaction is the conversion of the carboxylate group of pyruvate into a high-energy thio-ester.
pyruvate- + NAD+ + CoA-SH ¾® acetyl-CoA + CO2 + NADH + H+
Last modified March 17, 1997
Kent State University - Stark Campus
Department of Chemistry
This Page Written and Maintained by
Dr. Clarke Earley
email:
cearley@stark.kent.edu