
Physiological Chemistry Exam #1 Review
Chemistry 20284 - Physiological Chemistry
Spring 1997 - Exam #1 Review
Contents
- Chapter 2: The Polar Nature of Water (Acid/Base Chemistry)
- Chapter 3: Amino Acids and Peptides
- Chapter 4: The Three-dimensional Structure of Proteins
Chapter 2
- Electronegativity
- Know general trends. (For example, which is more electronegative, C, N, or O ?)
- Bond Polarity
- For the compounds we are interested in, assume H is "between" B and C on the periodic table. If two bonded atoms are next to each other on the periodic table, the bond is considered to be a non-polar bond. If the two bonded atoms are not next to each other on the table, the bond is considered to be a polar bond. An example question could be "Classify the following bonds as polar or non-polar (H-H, H-F, F-F, ...)".
- Lewis Structures
- Be able to draw Lewis structures for simple organic molecules. For example, draw the correct Lewis structures and identify the organic functional groups present in each of the following compounds. (Select any compound to see answer.)
Organic Functional Groups
Table 2.3 (pages 41 AND 42 of text) give the general structures and names of all of the organic functional groups we will use in this course. You will need to memorize and be able to draw Lewis structures for each of these.
Acid/Base Chemistry
For any chemical reaction reaction involving either a strong acid or a strong
base, the equilibrium shifts to give the lowest possible concentration of
the strong acid or strong base. Put another way, strong acids are very
reactive and tend to react with any base present (strong or weak) until all
of the strong acid or base is consumed.
For example, assume that HBr is added to a solution containing
CH3COOH (acetic acid) and CH3COO-1 (acetate
ion). Since HBr is a strong acid, it will react with any base present.
In this example, the acetate ion is the base and the following reaction
will occur:
CH3COO-1 + HBr
![]() CH3COOH + Br-1
CH3COOH + Br-1
The equilibrium for this reaction will lie as far to the right as possible, giving the lowest concentration of HBr. As a result of this reaction, the concentration of the weak base (CH3COO-1) decreases and the concentration of the weak acid (CH3COOH) increases.
- Strong Acids
- HCl, HBr, HI, HNO3, H2SO4, HClO4.
- Strong Bases
- LiOH, NaOH, KOH
- Conjugates
- Be able to label acid, base, conjugate acid, and conjugate base in any given reaction. (HW #3).
- Buffer
- Solution containing both weak acid and weak conjugate base. (See "Supplemental Homework - Chapter 2" for practice problems)
Equations
The following equations will be given. For the first equation, be able to calculate pH given [H+] and calculate [H+] given the pH (which is slightly harder).
pH = -log10([H+])
pH = pKa + log10([A-]/[HA])
The second equation shown is the Henderson-Hasselbalch equation. pKa values will be available (See Table 2.7 on page 50 of the text for typical values). This equation is typically used to solve buffer problems, where [HA] and [A-] are the concentrations of the weak acid and its conjugate base, respectively.
Chapter 3
amino acids
![]() peptides
peptides
![]() proteins
proteins
Be able to draw standard form of a general "L"-amino
acid: 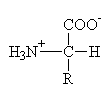
Given the structure of any amino acid, classify as either
non-polar (neutral), polar (neutral), acidic, or
basic.
Using the pKa values for each amino acid given in Table 3.2 and the structures, be able to draw the predominant structure of any amino acid at any pH value. To do this, you will need to know the structures of both the acid and base forms of the organic functional groups present in amino acids.
Peptides
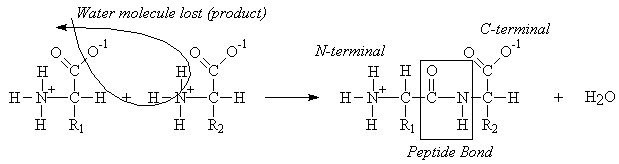
Given the amino acid sequence, draw the structure of any peptide. Be able to define and identify the N-terminal and C-terminal residues in any peptide. (Ex.: draw the structure and label the peptide bonds in Lys-Gly-Pro ).
Disulfide bridges
Formation of disulfide bridges occurs by the following reaction. Note that the thiol groups of the cysteine residues are oxidized in the course of this reaction. The reverse of this reaction can be accomplished by addition of the appropriate reducing agent. Be able to draw the structure of peptides (such as Cys-Ser-Cys) that contain this bridge.(R)-S-H + H-S-(R') ![]() (R)-S-S-(R') + 2 H+ + 2 e-
(R)-S-S-(R') + 2 H+ + 2 e-
Chapter 4
Structure of Proteins
- Primary
- Sequence of amino acid residues.
- Secondary
- 3-Dimensional arrangement of protein backbone.
- Tertiary
- 3-Dimensional arrangement of backbone and side chains (all atoms in chain).
- Quaternary
- Relationship between interacting polypeptide chains.
Interatomic Forces - determine which of these forces are present at each structure level.
- Covalent bonds (includes peptide bond and disulfide bridge)
- H-bonding (requires X-H, where X = F, O, or N)
- Electrostatic forces (attraction between electrically charges species)
- van der Waal's attractions (attraction between non-polar species)
Denaturing Proteins
Proteins can be denatured ("unfolded") using any one or combination of the following. Several of these act by attempting to strengthen the interaction of the protein with the solution, ultimately resulting in a situation where protein-solution forces are stronger, and thus more stable, than the protein-protein forces.
- heat
- changing pH
- detergents
- molecules that interfere with H-bonding
- reducing agents (used to break apart disulfide bridges).
Identify similarities and differences between
![]() -helices and
-helices and
![]() -sheets.
-sheets.
Collagen: -[X-Pro-Gly]-n triple helix structure. Strands strengthened by hydroxylation.
Myoglobin and Hemoglobin
Hemoglobin and myoglobin are responsible for
the transport (H) and storage (M) of oxygen in the body. Be able to identify
the important similarities and differences between myoglobin and hemoglobin.
(Primary structure, secondary structure, shape of protein, size of compounds,
prosthetic groups, function, O2 binding strength, etc.).
Know how each of the following factors influence O2 binding of hemoglobin:
- pH (Increasing concentration of [H+] decreases ability to bind O2).
- [CO2] (Addition of CO2 lowers pH, and therefor decreases ability to bind O2).
- [BPG] = 2,3-bisphosphoglycerate. (Decreases ability to bind O2 due to a structural change. Fetal hemoglobin has fewer positively charge sites to interact with BPG, which results in a less BPG and strong O2 binding).
![]() Return to Dr. Earley's
Home Page
Return to Dr. Earley's
Home Page
![]() Return to Physiological Chemistry
Home Page
Return to Physiological Chemistry
Home Page
Last modified February 5, 1997
Kent State University - Stark Campus
Department of Chemistry
Dr. Clarke Earley
