
Educational Materials for Organic Chemistry
Acid-Base Equilibria Tutorial
This tutorial will allow you to explore the effect of changing reaction conditions on the acid-base equilibrium reactions of bromoacetic acid, a simple organic acid. The initial solution consists of 0.1 moles of bromoacetic acid in 1 L of water adjusted to pH 7. Once you have completed your exploration into the effect of changing a variable, select the link at the bottom of future pages to go to the explanation about that variable's effect on equlibria.
Suggestion: You may want to try making a rough graph of each adjustable parameter versus the concentration of either the acid form (A) or the basic form (B) of bromoacetic acid. (or against any other parameter which seems to be changing in response to your choices!)
Table 1: Adjustable Values
| Previous pH | 7 | Current pH | 7 |
| Previous Temperature | 25 degrees C | Current Temperature | 25 degrees C |
| Previous Volume Solution | 1 L | Current Volume Solution | 1 L |
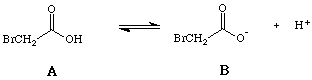
Table 2: Values Affected by Changes in Table 1
| Previous Concentration A | 7.98 x 10-6 M | Current Concentration A | 7.98 x 10-6 M | Change in Concentration A | 0.0 M |
| Previous Concentration B | 0.1 M | Current Concentration B | 0.1 M | Change in Concentration B | 0.0 M |
| Previous pKa | 2.902 | Current pKa | 2.902 | Change in pKa | 0.0 |






|
Slide 3 of 22
